
Neuralink's Vision-Restoring Brain Implant Earns FDA Breakthrough Status
October 22, 2024 – Neuralink, the pioneering neurotechnology company founded by Elon Musk, has achieved a significant milestone with its vision restoration device, Blindsight. The U.S. Food and Drug Administration (FDA) has granted the Blindsight device the prestigious “Breakthrough Device” designation, accelerating its development and review process.

Blindsight is engineered to restore vision to individuals who have lost their sight, even those without eyes or optic nerves. The device operates by implanting a microelectrode array into the visual cortex, which stimulates neurons to create visual experiences. Although the initial resolution is low, Neuralink aims to enhance it over time, potentially surpassing natural human vision and enabling users to perceive infrared, ultraviolet, or even radar wavelengths.
Elon Musk has expressed optimism about Blindsight’s potential, envisioning a future where people who have been blind from birth can see for the first time. Nevertheless, experts caution that while the breakthrough designation is promising, the device remains in its early developmental stages and will require extensive testing before it can fully restore sight.

The FDA’s Breakthrough Devices Program is intended to expedite the development and review of medical devices that offer more effective treatment or diagnosis for life-threatening or irreversibly debilitating conditions. This designation enables Neuralink to collaborate closely with the FDA, addressing any issues that arise during the premarket review phase.
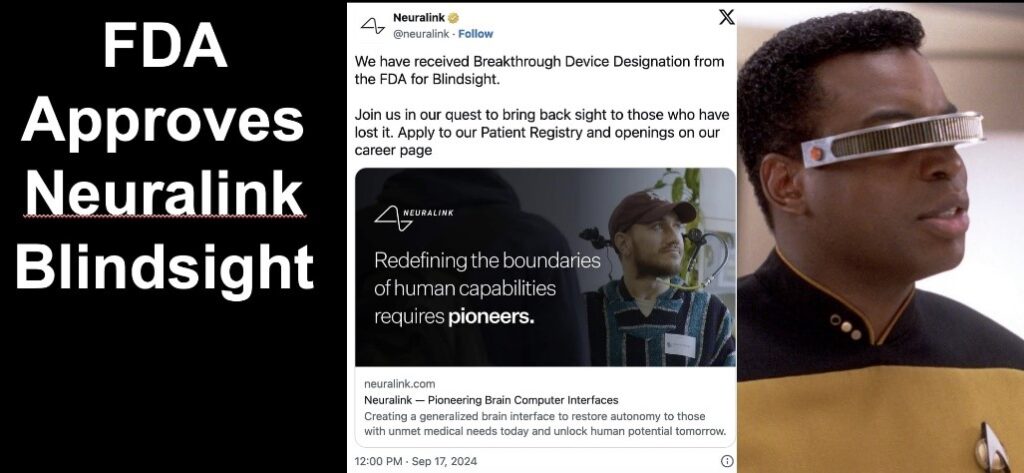
Neuralink’s Blindsight device signifies a substantial advancement in neurotechnology, offering renewed hope to millions of individuals with vision impairments. As development progresses, the world will be watching to see if this innovative technology can fulfill its transformative promises.



